Introduction
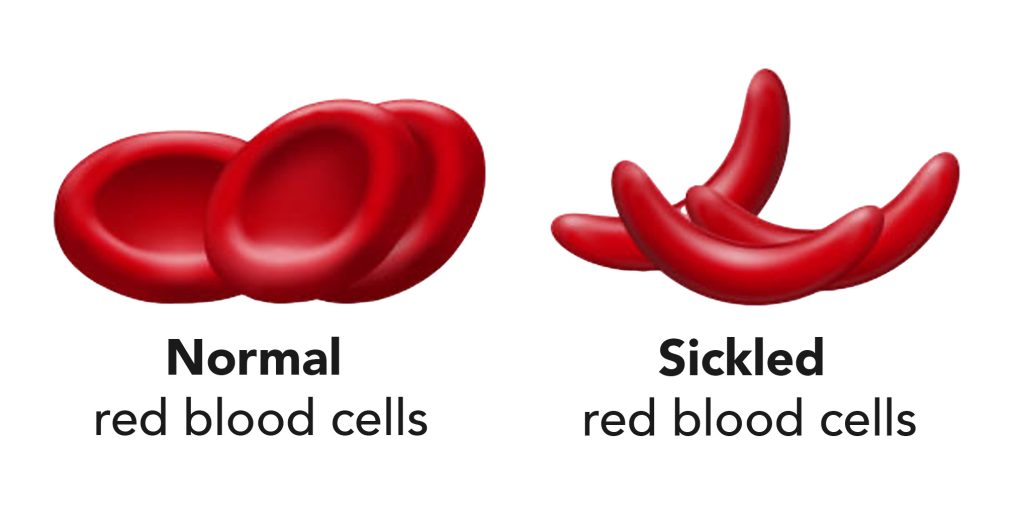
Children with sickle cell anemia often present to the emergency department with vaso-occlusive pain episodes (VOE), also referred to as pain crisis. In these patients, pain develops when red blood cells become sickle shaped, causing them to not be able to pass through the smaller blood vessels in joints and bones. This leads to severe pain.
How is VOE treated?
Currently, the cornerstone of therapy for managing pain in these children is opioid medications, which often carry with them, adverse effects, such as respiratory depression. They also are associated with a high risk of dependency. Despite technological advances, no effective treatments are available that target underlying mechanism of acute pain in children with sickle cell disease.
So how do we stop the high reliance on opiates for treatment of VOEs?
Studies have shown that decreased bioavailability of the amino acid L-arginine is associated with sickle cell disease. Also, it was recently discovered that arginine is converted to an opioid-like di-peptide (called Kyotorphin), suggesting that arginine is in fact acting like a pain medication [1]. This explains the opioid-sparing effect of arginine observed in past randomized control trials in the United States and Sub-Saharan Africa [2, 3].

Study: Sickle Cell Disease Treatment with Arginine (STArT)
STArT is the largest pediatric acute pain in sickle cell disease study, funded by the NIH, which seeks to determine if intravenous (IV) arginine is an effective treatment for acute pain among youth with sickle cell disease [4, 5].
Study question
Can IV arginine decrease duration of pain in VOE, and can it decrease opioid use in hospitalized children with sickle cell disease?
Study design
This is a multicenter, double blinded, placebo controlled, phase 3 trial, which will look to enroll 360 children presented to one of 10 study sites with VOE associated with sickle cell disease. The study began patient enrollment in 2021 and will continue through 2026. The study involves children who received an IV opioid for VOE and for the study. Study participants are randomized 1:1 to receive EITHER:
- Control therapy: A one-time placebo 2 mL/kg normal saline infusion followed by additional normal saline (1 mL/kg) three times per day.
- Intervention therapy: A single loading dose of IV L-arginine 200 mg/kg, followed by a standard dose of 100 mg/kg three times a day.
Results
As of May 2024, the study is 75% through its enrollments and 8+ months ahead of schedule!
“Also – a big event…The FDA just awarded orphan designation to arginine for the treatment of sickle cell disease (May 10, 2024), where I am the sponsor-investigator. This is an important step towards future regulatory approval if the STArT trial shows efficacy, to insure we can get this treatment to patients once the STArT study is completed.”
Dr. Claudia R. Morris, STArT principal investigator
Professor of Pediatrics and Emergency Medicine at Emory University
Caution
The main outcome measure for the study is time-to-crisis-resolution, which is directly related to length of hospital stay. As hospitals across the United States are very focused on quickly treating and discharging patients with sickle cell disease and VOE, (typically within less than 72 hours), this may impact the ability of this study to find a significant difference because of short length of hospital stay.

Why is this important for patients and caregivers
- VOE in children with sickle cell disease is associated with significant healthcare cost and impacts quality of life of children and young adults.
- No effective therapy outside of opioids and analgesics is currently available for acute VOE. Hydration is also used as an important part of treatment. Actually, we found that IV saline boluses worsened sickle cell pain in a PECARN study [6].
- Arginine has shown promise in prior investigations to be safe [7] and effective in reducing pain associated with VOEs [2-3].
Take Home Message
- For children with sickle cell pain, the treatment options are limited and often rely on use of opioid medications that are not without their side effects.
- Administration of the amino acid arginine is showing promise in treating sickle cell pain and reducing opioid use.
- Through the work of a large network like the Pediatric Emergency Care Applied Research Network (PECARN), the STArT study gives us a great opportunity to target a rare disease to see if arginine is effective in managing sickle cell pain and reducing opioid use.
References
- Korman R, Hatabah D, Brown LA, Harris F, Wilkinson H, Rees CA, Bakshi N, Archer DR, Dampier CD, Morris CR. Impact of Arginine Therapy on Kyotorphin in Children with Sickle Cell Disease and Vasoocclusive Pain. Blood Adv. 2024 Mar 25:bloodadvances.2023012209. doi: 10.1182/bloodadvances.2023012209. Epub ahead of print. PMID: 38527291.
- Morris CR, Kuypers FA, Lavrisha L, Ansari M, Sweeters N, Stewart M, Gildengorin G, Neumayr L, Vichinsky EP. A randomized, placebo-controlled trial of arginine therapy for the treatment of children with sickle cell disease hospitalized with vaso-occlusive pain episodes. Haematologica. 2013 Sep;98(9):1375-82. doi: 10.3324/haematol.2013.086637. Epub 2013 May 3. PMID: 23645695.
- Onalo R, Cooper P, Cilliers A, Vorster BC, Uche NA, Oluseyi OO, Onalo VD, Zubairu Y, Ayodele-Kehinde AU, Damilare OM, Figueroa J, Morris CR. Randomized control trial of oral arginine therapy for children with sickle cell anemia hospitalized for pain in Nigeria. Am J Hematol. 2021 Jan;96(1):89-97. doi: 10.1002/ajh.26028. Epub 2020 Nov 20. PMID: 33075179.
- Rees CA, Brousseau DC, Cohen DM, Villella A, Dampier C, Brown K, Campbell A, Chumpitazi CE, Airewele G, Chang T, Denton C, Ellison A, Thompson A, Ahmad F, Bakshi N, Coleman KD, Leibovich S, Leake D, Hatabah D, Wilkinson H, Robinson M, Casper TC, Vichinsky E, Morris CR; SCD Arginine Study Group and PECARN. Sickle Cell Disease Treatment with Arginine Therapy (STArT): study protocol for a phase 3 randomized controlled trial. Trials. 2023 Aug 17;24(1):538. doi: 10.1186/s13063-023-07538-z. PMID: 37587492
- Rees CA, Brousseau DC, Ahmad FA, Bennett J, Bhatt S, Bogie A, Brown KM, Casper TC, Chapman LL, Chumpitazi CE, Cohen DM, Dampier C, Ellison AM, Grasemann H, Hickey RW, Hsu LL, Lane PA, Bakshi N, Leibovich S, Patil P, Powell EC, Richards R, Sarnaik S, Weiner DL, Morris CR; SCD Arginine Study Group and PECARN. Adherence to NHLBI guidelines for the emergent management of vaso-occlusive episodes in children with sickle cell disease: A multicenter perspective. Am J Hematol. 2022 Nov;97(11):E412-E415. doi: 10.1002/ajh.26696. Epub 2022 Sep 5. PMID: 36054566
- Carden MA, Brousseau DC, Ahmad FA, Bennett J, Bhatt S, Bogie A, Brown K, Casper TC, Chapman LL, Chumpitazi CE, Cohen D, Dampier C, Ellison AM, Grasemann H, Hickey RW, Hsu LL, Leibovich S, Powell E, Richards R, Sarnaik S, Weiner DL, Morris CR; Sickle Cell Disease Arginine Study Group and the Pediatric Emergency Care Applied Research Network (PECARN). Normal saline bolus use in pediatric emergency departments is associated with poorer pain control in children with sickle cell anemia and vaso-occlusive pain. Am J Hematol. 2019 Jun;94(6):689-696. doi: 10.1002/ajh.25471. Epub 2019 Apr 29. PMID: 30916794.
- Reyes LZ, Figueroa J, Leake D, Khemani K, Kumari P, Bakshi N, Lane PA, Dampier C, Morris CR. Safety of intravenous arginine therapy in children with sickle cell disease hospitalized for vaso-occlusive pain: A randomized placebo-controlled trial in progress. Am J Hematol. 2022 Jan 1;97(1):E21-E24. doi: 10.1002/ajh.26396. Epub 2021 Nov 12. PMID: 34724240

